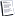 What kind of battery (cell) is this?
What kind of battery (cell) is this?
Bill Jeffrey wrote:
Sjouke Burry wrote:
Ha! That inside view is exactly the picture in my old
study books showing a standard voltage reference.
It might even still work.
If you want to test, use a high quality voltmeter,
and check its voltage.
DONT let it deliver any current, because it becomes
useless rather quickly.
OK, I understand that putting it under any kind of load will produce an
erroneous voltage. But if you do pull a few milliamps, is permanent
damage done, or will it recover (however slowly)? In other words, what
is the chemical reaction going on inside the cell? And how does it
manage to continue to produce a VERY constant voltage (plus or minus a
few microvolts!) for years and years, but will be damaged by pulling a
couple milliamps out of it for a few seconds?
Bill Jeffrey
You should reduce current load to microamps, and for as short a time
as possible.
I dont know the chemical side of things, but it was customary to
add a series resistance to the galvanometer to reduce load current to
the unbalanced circuit until balance was almost achieved.
Then the series resistor was shorted, and at he higher sensitivity
the balancing act was futher improved.
Milly_amps are definitely bad for the cell.
Any digital multimeter of current age are oke to measure cell voltage.
Almost all multimeters nowadays are several(10-200) megohms when measuring voltage.
|