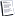 What kind of battery (cell) is this?
What kind of battery (cell) is this?
"Bill Jeffrey" wrote in message
...
Sjouke Burry wrote:
Ha! That inside view is exactly the picture in my old
study books showing a standard voltage reference.
It might even still work.
If you want to test, use a high quality voltmeter,
and check its voltage.
DONT let it deliver any current, because it becomes
useless rather quickly.
OK, I understand that putting it under any kind of load will produce an
erroneous voltage. But if you do pull a few milliamps, is permanent damage
done, or will it recover (however slowly)? In other words, what is the
chemical reaction going on inside the cell? And how does it manage to continue
to produce a VERY constant voltage (plus or minus a few microvolts!) for years
and years, but will be damaged by pulling a couple milliamps out of it for a
few seconds?
Bill Jeffrey
Bill, the standard cell is not a power source. Its sole purpose in life is to
provide a stable, low temperature coefficient source of voltage. It is designed
to be used intermittently, and to work into a very high resistance (ideally,
infinite resistance) load. It is used for calibration of very accurate
measuring instruments and systems. And yes, if you draw even a few milliamps
from the cell, it will be damaged.
I'm no chemist, and don't pretend to know anything about the chemical mechanism
that gives the standard cell its characteristics. If you're really that
interested in that, I suggest that you search the internet with Google to find
texts that describe the chemistry involved.
Search terms:
Weston saturated cell standard cell
--
Dave M
MasonDG44 at comcast dot net (Just substitute the appropriate characters in the
address)
"In theory, there isn't any difference between theory and practice. In
practice, there is." - Yogi Berra
|